For most chemists purifying organic reaction mixtures, normal-phase flash chromatography is the go-to technique. Why not, it is quick, relatively efficient, and can provide relatively high loading capacities if the separation is properly optimized. However, most of these same chemists rely on only two sets of solvents to perform these purifications…
-Hexane (or heptane) with ethyl acetate for low to moderate polarity reaction mixtures- -Methylene chloride (DCM) with methanol (sometimes with added base) for polar reaction mixtures
Frequently, the polar reaction mixtures being purified with DCM/MeOH become problematic with synthesized compounds either eluting too early, very late, or not separating at all. Besides those issues, DCM is not a safe solvent to use and does not lend itself to sustainable green chemistry, which currently is a polar subject and goal within many pharma and chemical companies.
So, with that in mind in this post I will provide some purification options by comparing different solvent systems for the purification of a polar reaction product.
The goal of reducing the use of harmful solvents in chemistry labs has been ongoing for many years. During my 22+ years at Biotage, this topic frequently occurs with customers asking what they can do to make their routine and non-routine purifications safer. Another frequent question I get asked is how to separate polar compounds that either do not elute or do not separate with DCM/MeOH mobile phase gradients. These are separate, but sometimes related issues.
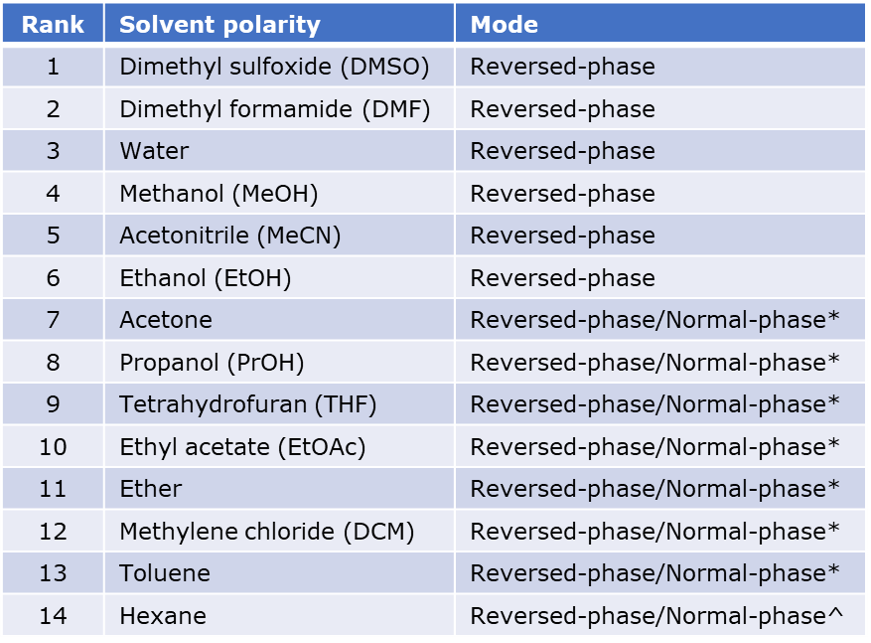
When purifying reaction mixtures, I have developed some guidelines based on reaction mixture solubility, Table 1. In this table, the more polar the reaction mixture solvent, the greater the need to use reversed-phase flash chromatography.
Table 1. Purification mode table based on solvent polarity
To explore various purification mobile phase options, I reacted hippuric acid and α-methylbenzylamine using a Biotage® Initiator+ microwave, Figure 1. The reaction solvent was acetonitrile, an aprotic, polar solvent unlikely to interfere with the synthesis.
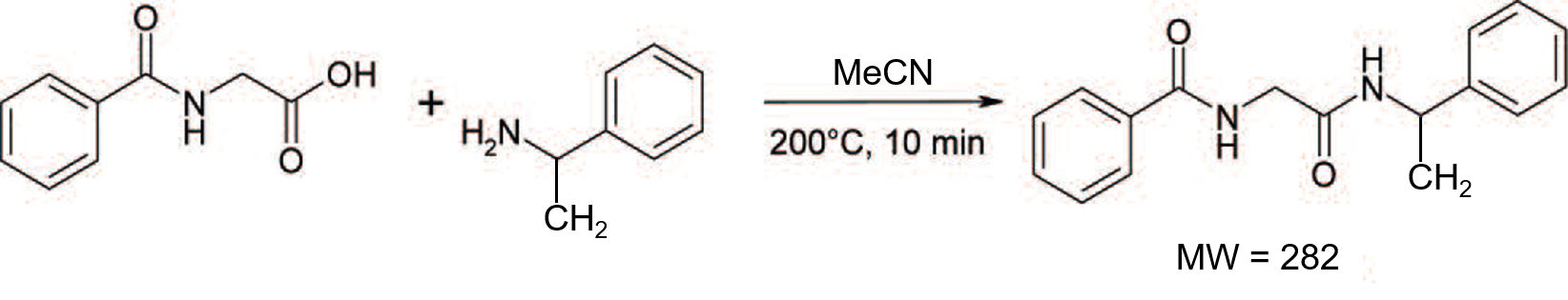
Figure 1. Reaction mixture used for this post.
The reaction mixture product was initially soluble in acetonitrile but began to slowly crystalize while cooling. To speed the crystallization process, I evaporated the acetonitrile with a Biotage® V-10 Touch and redissolved the solid in methanol (DCM did not work). I then performed TLC with three different solvent blends to evaluate their separation capability.
5% MeOH in DCM- 10% MeCN in DCM
- 40% (3:1 EtOAc/IPA) in hexane
The first solvent blend, MeOH/DCM, is very commonly used for these types of reaction mixtures. I personally prefer MeCN/DCM because its TLC RF values typically correlate to flash better than MeOH/DCM. The third solvent blend (40% 3:1 EtOAc/IPA in hexane) is based on the work of Emily Peterson and colleagues in their 2012 publication in Green Chemistry (Taygerly, Miller, Yee, & Peterson, 2012). For this publication they evaluated a number of mobile phase alternates to DCM/MeOH for pharmaceutical-like compounds and found a 3:1 ratio of EtOAc/EtOH, when used with heptane, to be the best alternative.
The TLC data from these three solvent mixtures show the product eluted with Rf values between 0.4 and 0.7. However, the separation of the product from some impurities was clearly best when using the EtOAc/IPA/hexane blend, Figure 2.

Figure 2. Reaction mixture TLC with different solvent systems. Left - 5% MeOH in DCM. Middle - 10% MeCN in DCM. Right - 40% (3:1 EtOAc/IPA) in hexane.
The resulting flash chromatography on my Biotage® Isolera Dalton 2000 showed the EtOAc/IPA/hexane gradient to be the most effective normal-phase method, fully removing the major by-product (+m/z 164) from the product (+m/z 283). Neither DCM/MeOH nor DCM/MeCN resolved the product from the by-product as effectively, Figure 3.
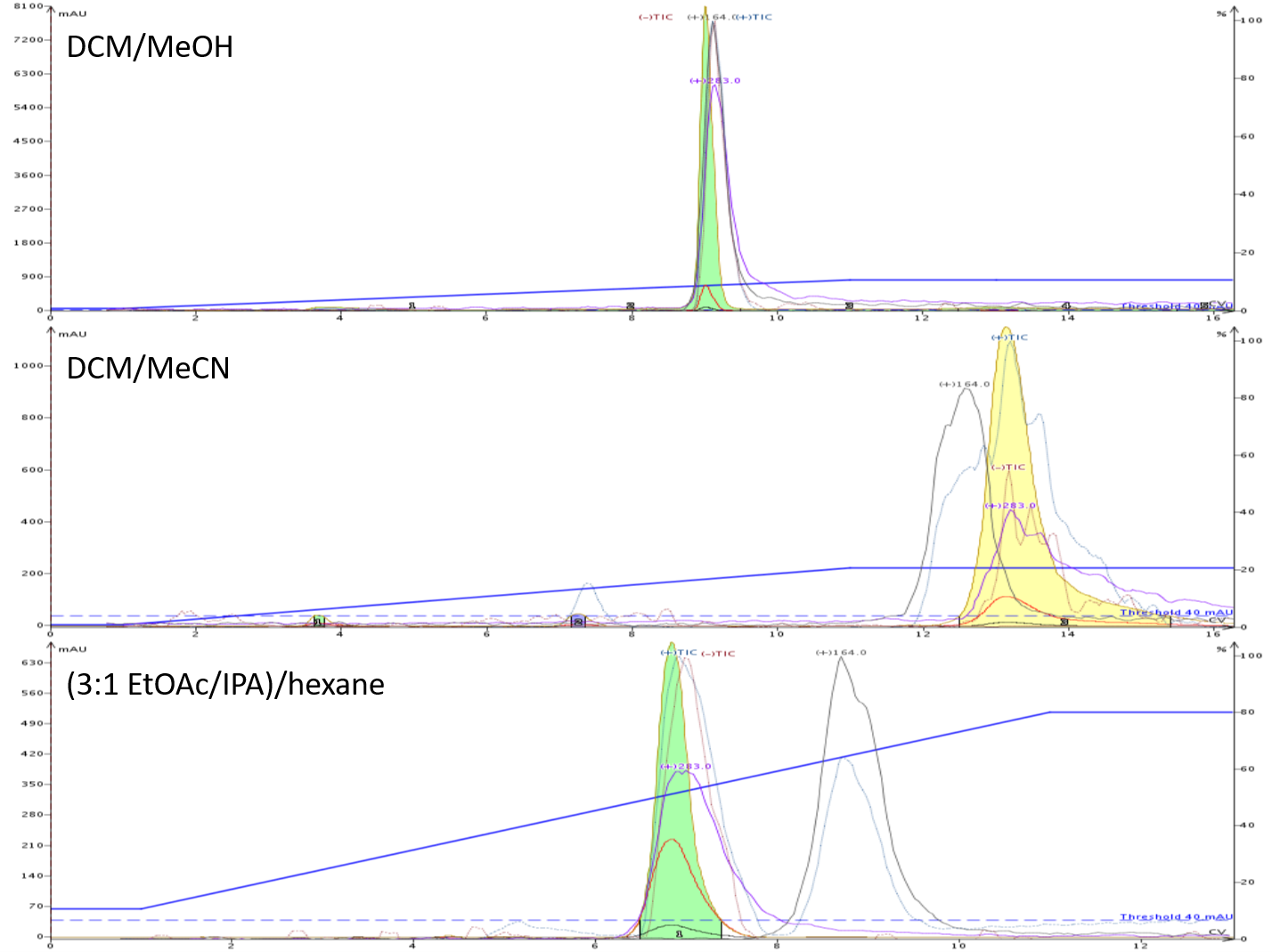
Figure 3. Reaction mixture flash chromatography comparison. Top - 0-10% MeOH in DCM (no separation of product and by-product). Middle - 0-20% MeCN in DCM (partial separation of product and by-product). Bottom - 10-80% (3:1 EtOAc/IPA) in hexane (full separation of product and by-product.
So, of the three normal-phase flash runs, the EtOAC/IPA/hexane method clearly provides the best purification and uses the most sustainable/green solvents.
An even more sustainable/green purification methodology can be used for this reaction mixture and most others as well – reversed-phase flash chromatography. Yes, you need to evaporate water from your product fractions, but it is not that big of a problem.
Though the reversed-phase columns are more expensive than silica columns, your purification results, not to mention the greenness and sustainability improvements, are likely to be much better with reversed-phase than normal-phase, Figure 4.
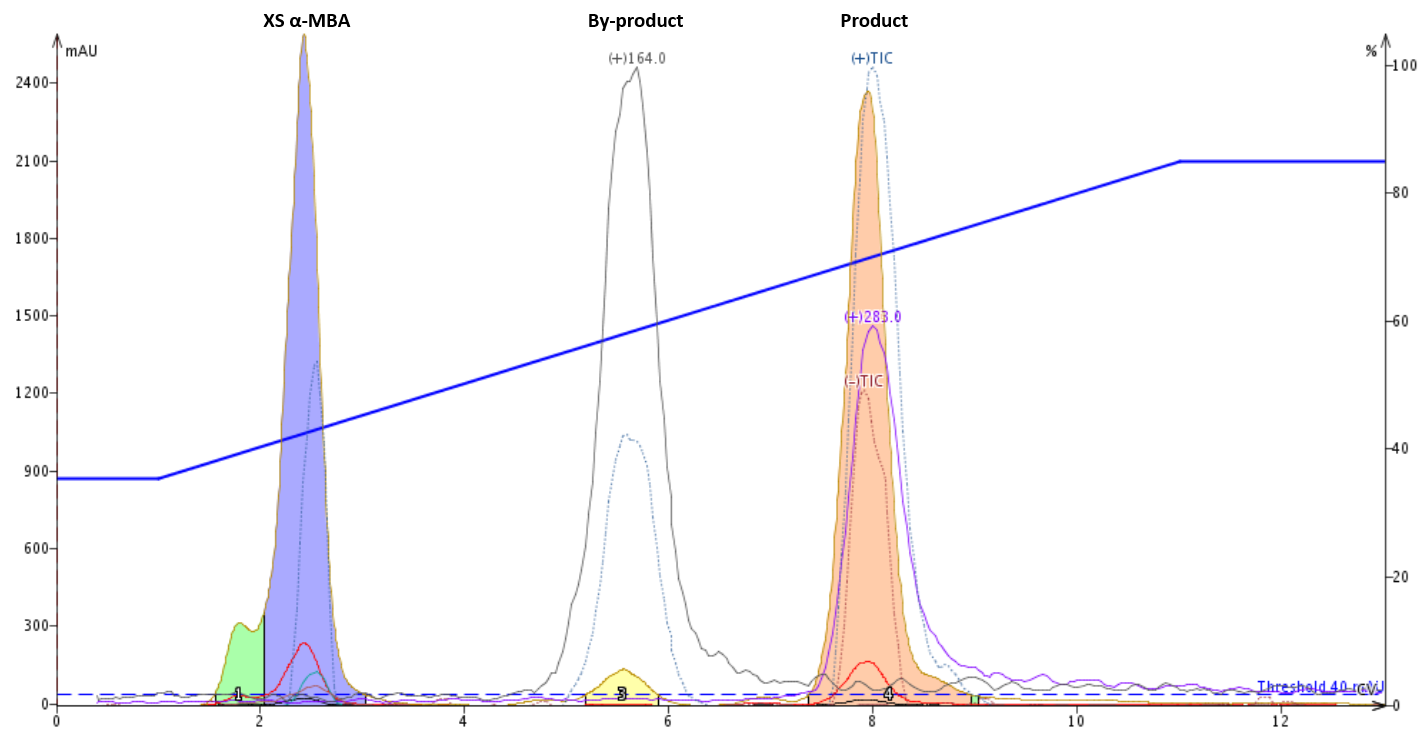
Figure 4. Reversed-phase reaction mixture purification (35-85% MeOH in water separates the product (pink peak from the major by-product and excess starting material.
Of all these options, reversed-phase is the most sustainable due to the generation of less waste...
- -Fewer organic solvents to dispose
- -Fewer plastic columns to dispose (reversed-phase columns are re-usable)
So, two options are available as alternatives to DCM/MeOH purification methods…
- -Normal-phase with the weak solvent hexane or heptane and the strong solvent 3:1 EtOAc/EtOH or 3:1 EtOAc/IPA
- -Reversed-phase
It’s your choice.
For more information on reversed-phase flash chromatography, please download our whitepaper (UI468).

 Organic Workflow
Organic Workflow Peptide Workflow
Peptide Workflow Scale-Up Flash Purification
Scale-Up Flash Purification  Sample Preparation
Sample Preparation Biomolecule Purification
Biomolecule Purification Oligo synthesis
Oligo synthesis Scavengers and Reagents
Scavengers and Reagents Service & Support
Service & Support Accessories & Spare parts
Accessories & Spare parts Investors
Investors Reports & News
Reports & News The Share
The Share Corporate Governance
Corporate Governance Calendar
Calendar Sustainability
Sustainability Our Offering
Our Offering Our History
Our History Our Locations
Our Locations Leadership
Leadership