An interesting question for synthesis chemists as the need for reversed-phase flash chromatography for both intermediates and final compounds increases. Historically, normal-phase flash chromatography has been the “go-to” purification tool for synthetic intermediates. Today, however, intermediates and final products are increasingly polar. These polar reaction mixtures cause purification challenges in both separation and recovery with normal-phase (silica) thus requiring use of reversed-phase flash chromatography.
Reversed-phase chromatography, for those unfamiliar with the technique, uses a column filled with a hydrophobic stationary phase. The solvent used with reversed-phase are typically water miscible, the most popular being methanol and acetonitrile. Other, less popular reversed-phase solvents include tetrahydrofuran and acetone.
While normal-phase allows the use of a wide range of solvents with different selectivity options, reversed-phase is limited. That being said, selectivity differences are sometimes seen in reversed-phase when the organic solvent is changed, which can improve the separation of some compounds – a major focus of this post.
Other variables in deciding to use methanol or acetonitrile include…
- -Cost
- -Viscosity
- -UV absorption
Cost
If you ever purchased solvent you likely have noticed that methanol is far less expensive than acetonitrile, 4x less expensive, certainly a compelling reason justifying its use for everyday reversed-phase flash chromatography.
While saving money on solvent makes sense, the other parameters mentioned above should also be considered.
Viscosity
Water is polar and fairly viscous, at least compared to most other solvents. When blended with other solvents the viscosity increases with increasing organic (up to ~50%) which, in turn, elevates backpressure, Figure 1.
As the organic solvent percentage increases past 50%, the viscosity and pressure decrease. This is important as increasing pressure during flash purification can restrict flow rate, increasing purification time and sometimes cause system overpressure. If this is a concern, acetonitrile is the better option.
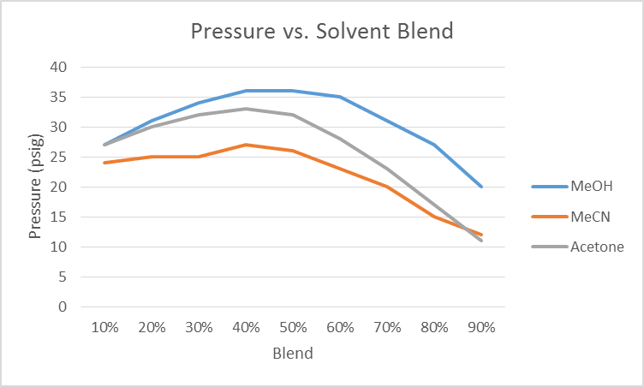
Figure 1. Pressure vs. % organic solvent in water. The pressure reaches a maximum as the % organic nears 50% for each solvent.
UV absorption
Most solvents absorb UV light to some extent. However, water and acetonitrile are totally UV transparent above 198 nm, the lower limit for most commercial flash system UV detectors. Methanol, tetrahydrofuran, and acetone all absorb UV light which can hinder compound detection. Table 1 lists the common reversed-phase solvents and their UV cut-off, which is the wavelength at which UV light is not longer absorbed[1].
Table 1. Typical reversed-phase chromatography solvents and their UV cut-off.
Solvent UV cutoff (nm)
Acetonitrile 190
Water 191
Methanol 210
Tetrahydrofuran 220
Acetone 330
Selectivity
Though I have discussed selectivity previously, I feel it is important to address this again to reemphasize the benefits derived from method selectivity optimization. After all, without selectivity there is no separation!
Not all compounds will show altered elution patterns when evaluating different solvent blends. However, if you find you have a pair of compounds poorly resolved with one solvent, why not quickly evaluate another solvent?
To help illustrate the benefits achievable by evaluating different solvents, I have a particularly interesting example. The crude material being purified was a natural product extract that I attempted to first purify using reversed-phase with a 60-100% methanol-water gradient, Figure 2. This gradient provided some separation of the four most abundant compounds but could not fully resolve one compound with a +m/z of 302.8 from another with +m/z 272.8 using a Biotage® Isolera Dalton 2000 flash system and a 12-gram Biotage® Sfär C18 column.
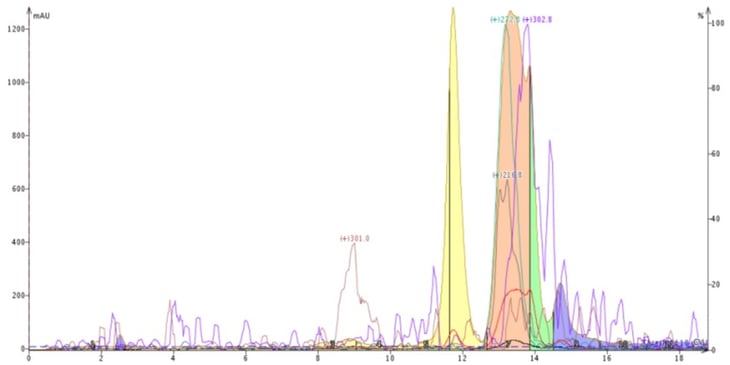
Figure 2. Reversed-phase purification using a water-methanol gradient. The in-line mass detector confirmed an incomplete separation of the compounds with +m/z 272.8 and +m/z 302.8 (pink peak).
In an attempt to improve the chromatography I swapped methanol for acetonitrile and ran a 40-100% acetonitrile-water gradient. To my enjoyment acetonitrile provided different selectivity, eluting the compound with +m/z 302.8 before the compound with +m/z 272.8 and generating a better overall separation, Figure 3.
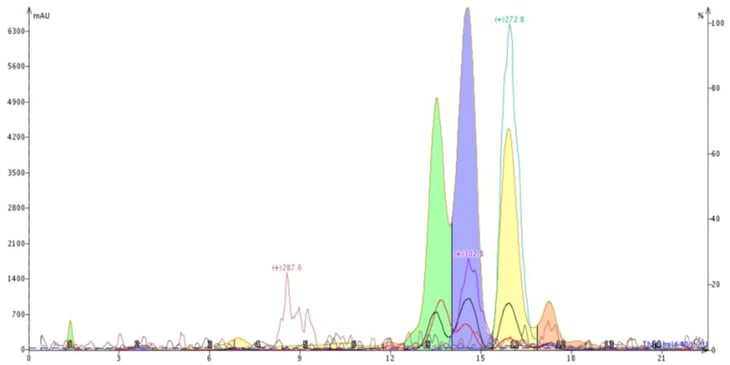
Figure 3. Reversed-phase purification of the natural product extract using a water-acetonitrile gradient altered the elution order and improved the purification. The compound with +m/z 302.8 (blue peak) now elutes earlier than the compound with +m/z 272.8 (yellow peak).
Does this data suggest that acetonitrile should be the preferred solvent in reversed-phase? No, though it does support the suggestion that both methanol and acetonitrile should be evaluated when creating a reversed-phase flash purification method.
For more information on reversed-phase method development please download my post on converting HPLC methods to flash chromatography.
[1] Dean, J. A. Lange’s Handbook of Chemistry, 15th ed.; McGraw-Hill: New York, 1999, p 11.16

 Organic Workflow
Organic Workflow Peptide Workflow
Peptide Workflow Scale-Up Flash Purification
Scale-Up Flash Purification  Sample Preparation
Sample Preparation Biomolecule Purification
Biomolecule Purification Oligo synthesis
Oligo synthesis Scavengers and Reagents
Scavengers and Reagents Service & Support
Service & Support Accessories & Spare parts
Accessories & Spare parts Investors
Investors Reports & News
Reports & News The Share
The Share Corporate Governance
Corporate Governance Calendar
Calendar Sustainability
Sustainability Our Offering
Our Offering Our History
Our History Our Locations
Our Locations Leadership
Leadership
