In a previous post, I evaluated how flow rate can impact my purification efficiency using flash chromatography. I noticed though, that my peptide eluted significantly later with high mobile phase flow rates. I hypothesized that the increased pressure (caused by higher flow rates) was driving the compound further into the pores, increasing the overall interaction with the stationary phase and causing the increased retention. We know that the particle size and particle pore size impact resolution and purification efficiency, so how does flow rate play a role with a different stationary phase?
In today’s post I’ll evaluate several flow rates using a reversed phase stationary phase material with slightly larger diameter particles that possess significantly smaller pores.
In the previous experiments, I purified an amphipathic 18 amino acid peptide using a Biotage® SNAP Bio C18 cartridge. I’ll repeat those experiments in today’s post using a SNAP Ultra C18 cartridge. While the packaging looks similar, there are some important differences detailed in Table 1.
| Cartridge Name | Particle Size | Pore Size | Column Volume |
|---|---|---|---|
| SNAP Ultra C18 | 25 um | 100A | 15 mL |
| SNAP Bio C18 | 20 um | 300A | 17 mL |
Table 1. Comparison of stationary phase medias used in these experiments.
My previous work compared the purification efficiency of these two cartridges, but only at a single flow rate. After the initial flow rate work was completed I began to wonder if the effects would be as significant with the SNAP Ultra C18 cartridges as well. For these experiments I loaded about 50 mg of peptide dissolved in 300 uL DMSO onto a pre-equilibrated SNAP Ultra C18 cartridge and ran a gradient from 20 to 70% MeCN using flow rates of 12, 25, or 50 mL/min, Figure 1.
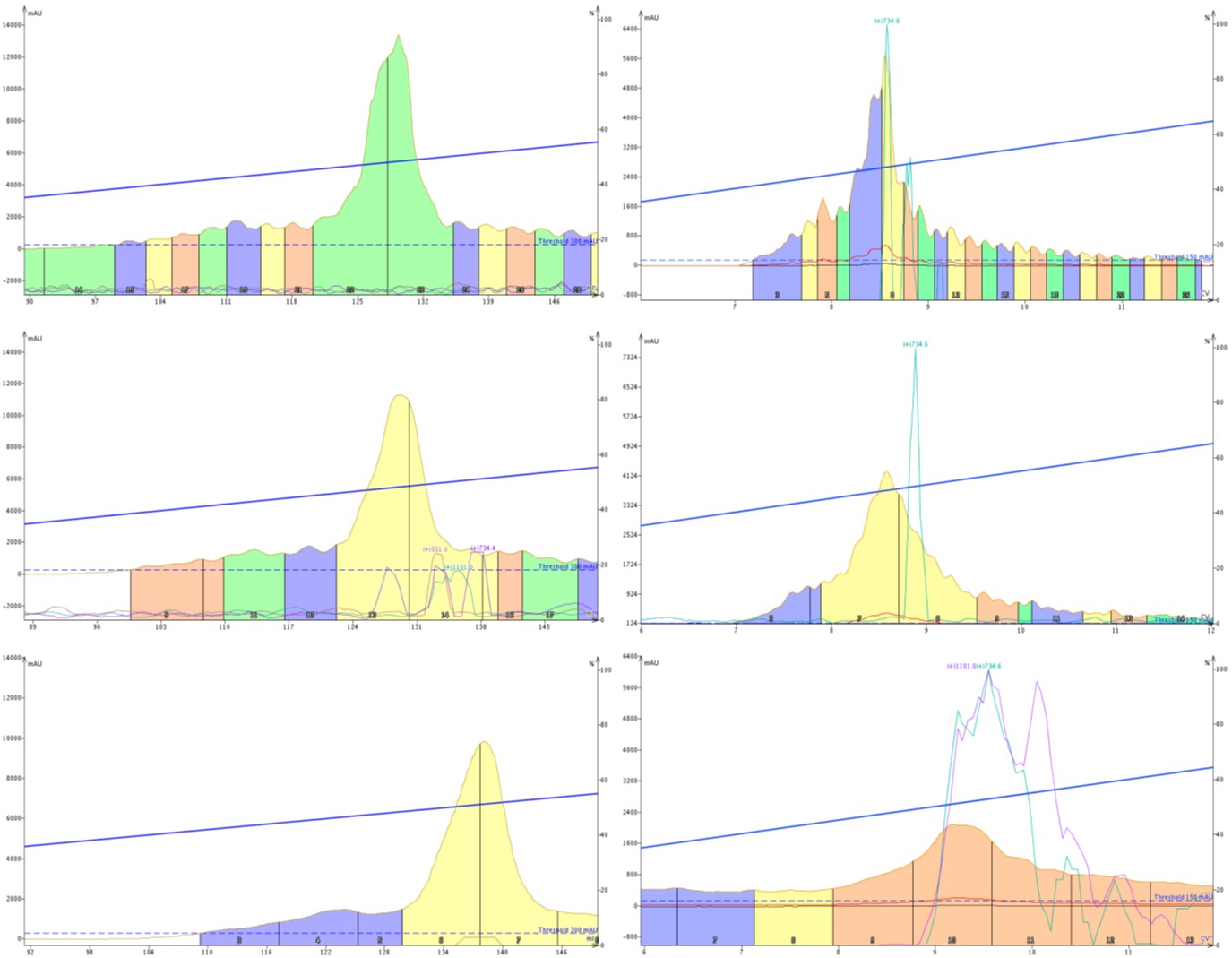
Figure 1: Purification of 18A using SNAP Bio C18 (left) or SNAP Ultra C18 (right) cartridges. The purification was completed using identical gradients but flow rates of 12 (top), 25 (middle) or 50 mL/min (bottom).
I decided to include the SNAP Bio C18 chromatograms for easy reference and the first thing I notice is a significant increase in the baseline fluctuation with the SNAP Ultra C18 cartridge, particularly at low flow rates. Just as with the SNAP Bio C18 cartridge though, the pulsation decreases at higher flow rates and is essentially eliminated at 50 mL/min.
What has changed significantly though is resolution. I have done some other purification work with this particular batch of peptide and I know there is an early eluting impurity peak, Figure 2. With the slowest flow rate, you can sort of convince yourself that that peak is still evident. Sort of. But as the flow rates increase, the resolution of this impurity decreases, to the point that it is completely absorbed into the product peak, decreasing the final purity and effectively wasting my time.
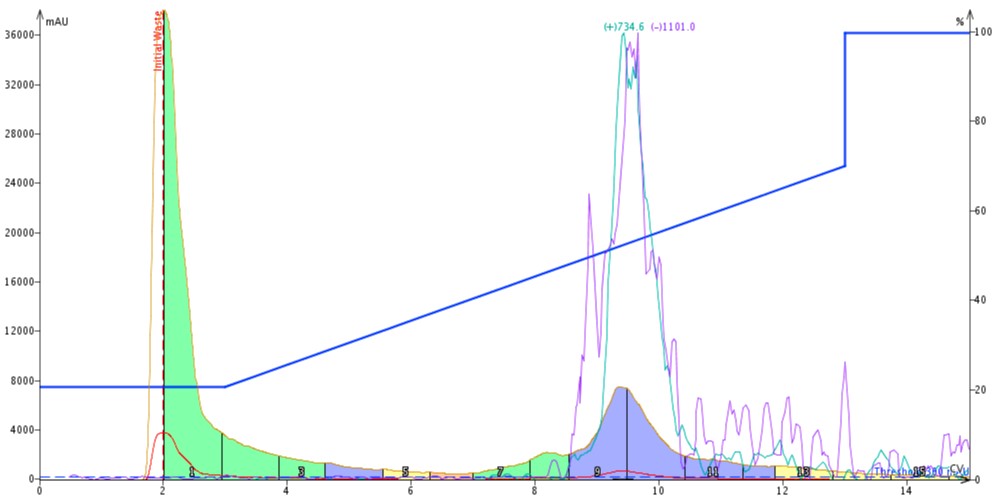
Figure 2: Purification of 100 mg 18A using a SNAP Bio C18 cartridge. A leading impurity (green hump) is clearly evident in this purification effort.
If we look at the separation efficiency, it becomes abundantly clear that the purification using SNAP Ultra C18 is significantly less efficient that when performed with SNAP Bio C18, Table 2. Most significant is the dramatic decrease in apparent theoretical plates that occurs as flow rate is increased, compromising the purification. Using this type of cartridge, I would certainly invest more time running a slower gradient to ensure that the purification was actually successful in the first attempt.
| Flow rate (mL/min) | Retention volume (mL) | Peak width at half max (mL) | Theoretical plates per column | Theoretical plates per meter |
|---|---|---|---|---|
| 12 | 145.5 | 5.5 | 3,877 | 70,493 |
| 25 | 146 | 13.5 | 648 | 11,781 |
| 50 | 157 | 22 | 282 | 5,130 |
I usually recommend using a higher flow rate because the purification time shortens, but it becomes a judgement call at this point. The larger particles and smaller pores of the SNAP Ultra C18 increase the available surface area (proportional to loading capacity) of the cartridge, potentially reducing the number of injections and allowing the use of a smaller cartridge (less solvent consumed). However, the improved column efficiency and overall purification observed with the SNAP Bio C18 makes me lean towards this stationary phase. I’d rather run 2 injections and successfully purify my peptide to a high degree of purity than only take 1 injection and have a less pure compound at the end.
Can flash chromatography really impact your overall peptide workflow, increasing the number of compounds delivered as final product? Follow the link below to find out!

 Organic Workflow
Organic Workflow Peptide Workflow
Peptide Workflow Scale-Up Flash Purification
Scale-Up Flash Purification  Sample Preparation
Sample Preparation Biomolecule Purification
Biomolecule Purification Oligo synthesis
Oligo synthesis Scavengers and Reagents
Scavengers and Reagents Service & Support
Service & Support Accessories & Spare parts
Accessories & Spare parts Investors
Investors Reports & News
Reports & News The Share
The Share Corporate Governance
Corporate Governance Calendar
Calendar Sustainability
Sustainability Our Offering
Our Offering Our History
Our History Our Locations
Our Locations Leadership
Leadership