Orthogonal side chain protecting groups, particularly for Fmoc-based solid phase peptide synthesis, are growing not only in diversity, but also in popularity. These protecting groups enable post-synthesis chemistry while the peptide is still on resin, often times increasing efficiency, decreasing side reactions, and generally simplifying the overall process.
I've already done some work with many of the commercially available orthogonally protected amino acids including allyl and alloc, Acm, and ivDde for a variety of downstream applications. In today's post, I'll discuss some work optimizing the removal of a 4-methoxytrityl (Mmt) group from cysteine side chains.
After thinking about this off and on for a few months, I remembered from my past proteomics work that proteins are typically denatured, then cysteine residues alkylated prior to trypsin digestion and mass spec analysis. Often times, this reaction is completed in solution using aqueous conditions, but I was able to identify a previously published on-resin protocol to get me started. Of all the cysteine side chain protecting groups, Mmt is the one I've used most frequently. However, I've struggled with how to measure the deprotection efficiency. As you may know, Mmt is removed with low concentrations of TFA however, any orthogonal deprotection can be masked by the penultimate peptide cleavage from the resin.
The nice thing about alkylating free Cys residues is that this is an irreversible reaction, enabling me to quickly identify how much of the Mmt protecting group was removed in the deprotection conditions and not the final cleavage and global deprotection.
For these experiments optimizing Mmt removal conditions I first synthesized oxytocin, a short peptide that contains two cysteine residues with my Biotage® Initiator+ Alstra™ peptide synthesizer using Fmoc-Cys(Mmt)-OH for both cysteine residues. With on-resin alkylating conditions in hand, I divided the resin from a single synthesis into roughly equal quantities and evaluated a variety of reaction conditions with 2% TFA in DCM and 5% TIS to scavenge the released Mmt groups, Table 1.
| Experiment | Reaction time (min) | Reaction Iterations |
| 1 | 10 | 5 |
| 2 | 5 | 5 |
| 3 | 1 | 5 |
| 4 | 10 | 3 |
Table 1. Reaction conditions evaluated for removal of Mmt protecting groups from oxytocin while on resin.
Interestingly, in all of the different protocols I observed doubly alkylated, singly alkylated, and oxytocin with no alkylation, Figure 1.
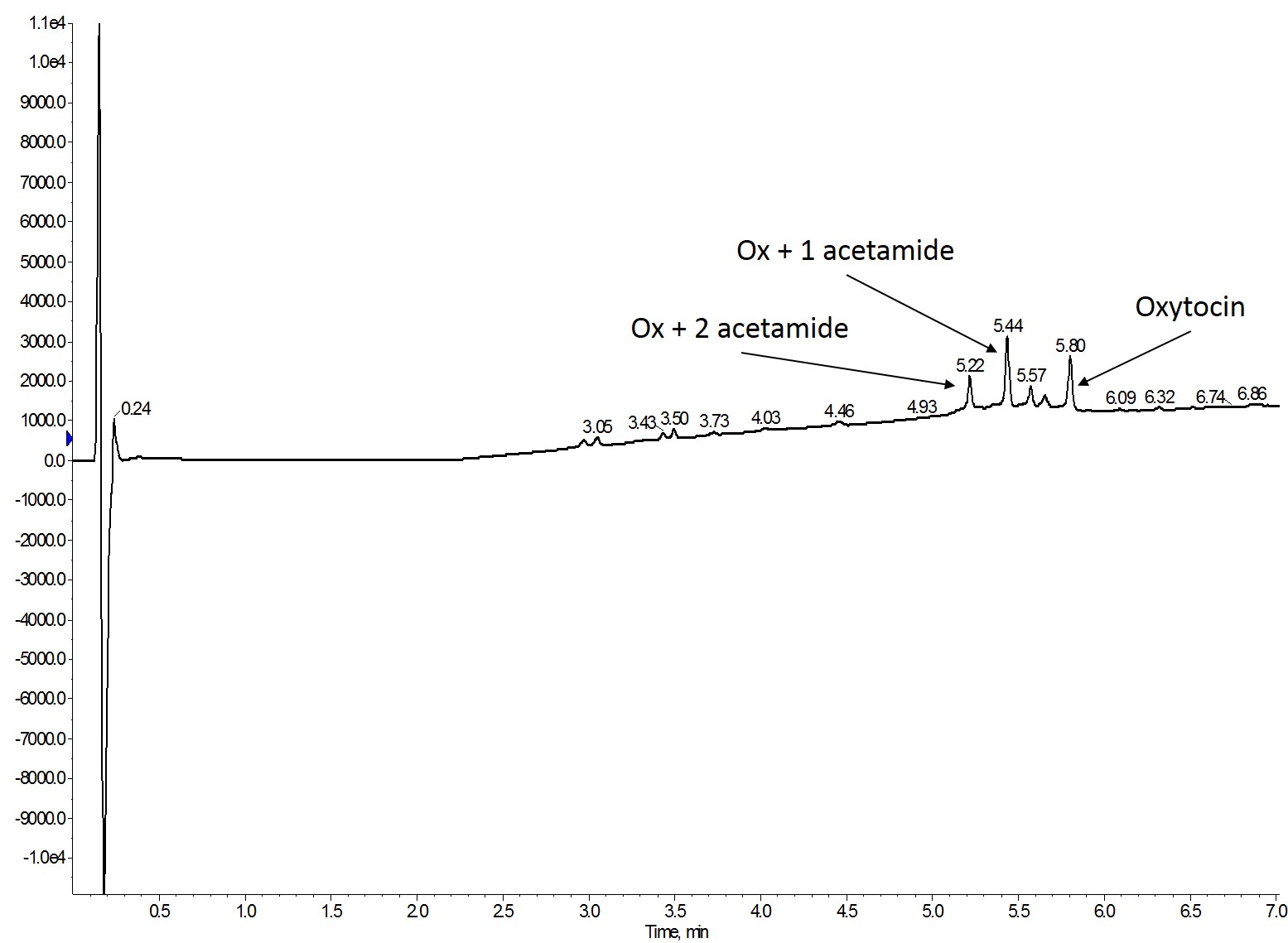
Figure 1: Representative analytical HPLC chromatogram observed for all Mmt removal conditions. For these studies, oxytocin is a linear, fully reduced peptide in all peaks.
This bears the question though - does this alkylation reaction require additional optimization or are the deprotection strategies really that inefficient? My assumption at this point is that the alkylation reaction requires some additional optimization for this particular peptide as I've used one of these methods in the past for on-resin cysteine oxidation and found complete conversion by analytical HPLC chromatography.
Regardless, this mixture complicates my analysis needed to determine the most efficient deprotection protocol. Ultimately, I decided to compare the total alkylated peptide content (singly plus doubly alkylated) to the unmodified oxytocin content as a percentage of the loaded sample amount, Table 2.
|
Experiment
|
Doubly Alkylated (µg)
|
Singly alkylated (µg)
|
unmodified oxytocin (µg)
|
total crude peptide loaded (µg)
|
percent alkylated
|
| 1 | 0.313 | 0.635 | 0.514 | 1.77 | 53.6 |
| 2 | 0.568 | 1.043 | 1.082 | 3.33 | 48.8 |
| 3 | 0.275 | 0.362 | 0.935 | 1.99 | 32 |
| 4 | 0.0101 | 0.018 | 0.0207 | 0.0705 | 40 |
Table 2. Total alkylated oxytocin content relative to total amout of crude sample injected for analytical HPLC evaluation.
Based on these results, it is clear that a 10 min reaction time repeated a total of 5 times yields the greatest percentage of Mmt deprotected cysteine side chains. Typically, this (and other) orthogonal protecting groups are used to enable additional post-synthesis chemistry and for that to be successful, this percent deprotection will certainly have to get closer to 100% completion; but this is certainly a good starting point! To further optimize this reaction, I'll likely increase my TFA concentration before increasing iterations or reaction time.
I am still curious how much further optimization would be necessary to fully alkylate the cysteine residues on resin, although this is somewhat less interesting from a functional peptide perspective. Stay tuned for a future discussion!
To learn more about how Mmt and other orthogonal protecting groups are used to prepare peptides with one or more disulfide bonds, follow the link below.

 Organic Workflow
Organic Workflow Peptide Workflow
Peptide Workflow Scale-Up Flash Purification
Scale-Up Flash Purification  Sample Preparation
Sample Preparation Biomolecule Purification
Biomolecule Purification Oligo synthesis
Oligo synthesis Scavengers and Reagents
Scavengers and Reagents Service & Support
Service & Support Accessories & Spare parts
Accessories & Spare parts Investors
Investors Reports & News
Reports & News The Share
The Share Corporate Governance
Corporate Governance Calendar
Calendar Sustainability
Sustainability Our Offering
Our Offering Our History
Our History Our Locations
Our Locations Leadership
Leadership