This is a question being asked of my colleagues and me more and more frequently, especially in pharma accounts. Why? Well, you are familiar with the adage – Time is Money, right. Well this really applies to them. A new molecular entity (NME) created as a pharmaceutical can take up to a decade and a billion dollars to bring to market. Granted, the biggest costs are in the clinical trials but the synthetic route and the time to discover and make the compound – and purify it – plays a major role within drug discovery and development. This timeline is not helped by the ever increasingly difficult-to-synthesize compounds being investigated as drug candidates today.
With that in mind, this post focuses on ways to speed the purification process without sacrificing purity and yield.
When I talk about flash chromatography with other chemists, I ask them why they use it and what is most important for them – compound purity, recovered yield, or speed. In the past, the answer has typically been “for intermediates I need 80-85% purity and enough material to run the next synthetic step”. Speed was never a factor. For final compounds ready for submission/biological testing, the answer changed to I need high purity (98%+), followed by yield; again purification speed was not important.
Today, however, we are seeing a need by chemists to fail earlier so that they can succeed faster with a compound that meets structure activity relationship (SAR) and biological activity goals. The faster they find the "right" compound with all the desired characteristics, the more research dollars (man-hours and solvent costs) are saved as the compound moves more quickly to downstream areas of scale-up and testing.
There is a fundamental challenge chromatographically with trying to optimize a purification method for purity, yield, and speed. With most flash columns, the faster the flow rate (reduces time), the worse the separation. This is due to increases in diffusion, dispersion, and other phenomena I will not get into at this time but you can research yourself, if interested, by clicking on the links above.
You see, speed, yield, and purity are inter-related and you can only really optimize on two of the three as illustrated in the Chromatographer’s Triangle, Figure 1. Sure, various techniques have been developed to address the trade-offs but the compromises have had limitations.
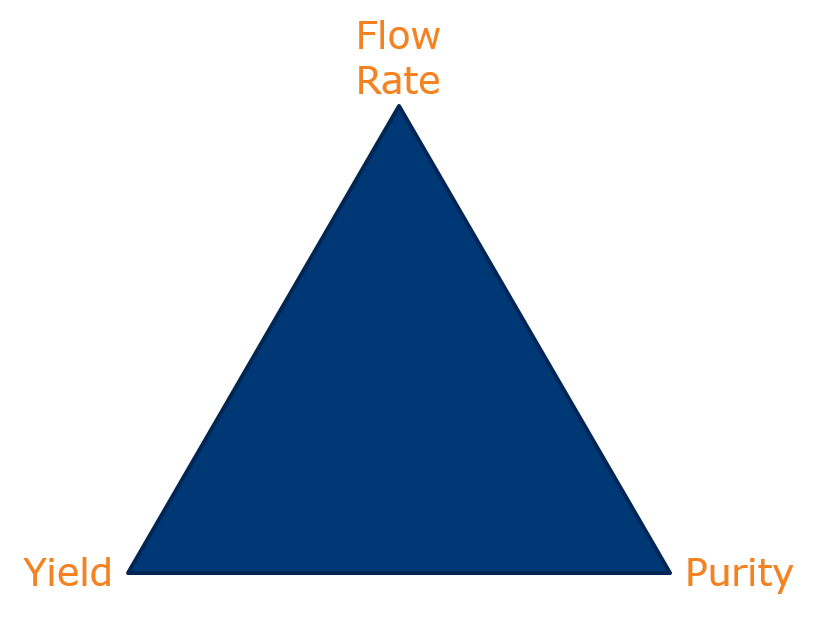
Figure 1. The Chromatographer's Triangle represents the tradeoffs between flow rate (speed), yield, and purity.
Fortunately, those researching and creating chromatography products are finding not only better method optimization techniques but also improved products – both hardware and columns.
A case in point is the recent development of a new flash column line called Biotage® Sfär columns. What is new, different, and impactful to you as a chemist is the media, a 20 µm spherical silica with about 750 m²/g surface area.
Most of you know the relationship between particle size and separation efficiency – smaller particles = higher separation efficiency = higher purity fractions or better purification yields and the potential to reduce column size (which saves solvent and, potentially, time). The problem with this thinking is that a 50% reduction in particle size provides only a 41% increase in separation or resolution. A 41% resolution improvement is good but this alone will not provide the needed efficiency gains in the drug discovery lab. However, the combination of a small particle size and high surface area (increases loading capacity) silica enables a fundamental change in drug discovery flash chromatography thinking.
An increase in silica surface area from the typical ~500 m²/g to ~750 m²/g can double the loading capacity for silica with the same particle size. A smaller particle (20 µm) silica with a 750 m²/g surface area increases loading capacity 3x to even 4x over traditional silica and now provides chemists a way to speed their flash purification by using a column one-half to potentially one-third of the typical size for the given sample load.
I had the opportunity recently to see this for myself. I purified a 100 mg of a mixture of hard-to-separate parabens (methyl and butyl) using a 10 gram Biotage® KP-Sil column, which is packed with traditional flash silica - 40-63 µm, ~500 m²/g. The separation was actually quite decent but the programmed run required 195 mL of solvent and 7.2 minutes to complete, Figure 2.
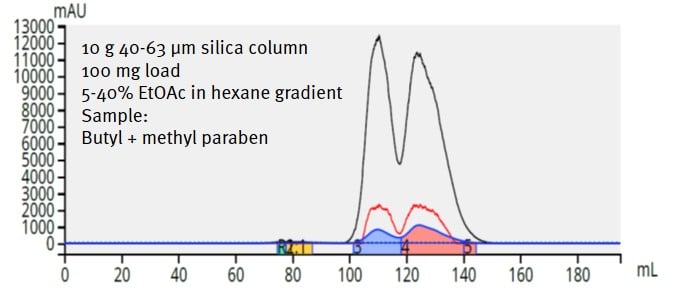
Figure 2. Separation of 100 mg of paraben mix using a 10 gram flash column packed with traditional 40-63 µm, 500 m2/g silica provides a good separation, but consumes 195 mL of solvent and 7.2 minutes.
I then purified the same amount of the paraben mix using a 5-gram Biotage® Sfär HC column (20 µm, 750 m²/g) and achieved the same separation (although with more retention due to the increased surface area). Still, using the 5 g column required less solvent (only 117 mL) and time (only 6.5 minutes) than the 10 gram column, Figure 3.
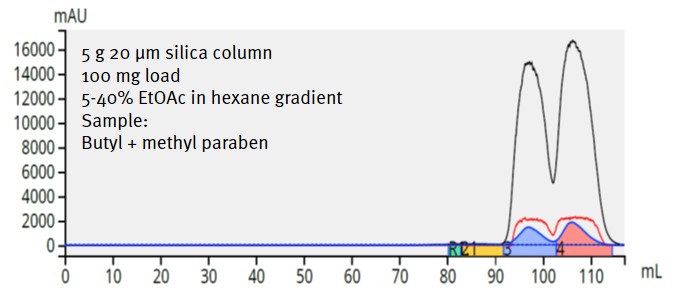
Figure 3. Separation of 100 mg of paraben mixture using a 5 gram, 20 µm, 750 m2/g silica column provides a separation equivalent to the larger 10 gram column but consumes only 117 mL and requires only 6.5 minutes to complete.
Well, the data does indeed show that a column with ½ the amount of silica (the 5g column) purified the same amount of sample as the 10 gram column, and did it 11% faster using 40% less solvent. I find that quite impressive.
So, this information tells me that you can maximize purity, yield, and speed if you select the right column and media. Sounds like the correct prescription for a drug discovery program.
For more information on flash chromatography, I invite you to listen to my webinar on this subject.

 Organic Workflow
Organic Workflow Peptide Workflow
Peptide Workflow Scale-Up Flash Purification
Scale-Up Flash Purification  Sample Preparation
Sample Preparation Biomolecule Purification
Biomolecule Purification Oligo synthesis
Oligo synthesis Scavengers and Reagents
Scavengers and Reagents Service & Support
Service & Support Accessories & Spare parts
Accessories & Spare parts Investors
Investors Reports & News
Reports & News The Share
The Share Corporate Governance
Corporate Governance Calendar
Calendar Sustainability
Sustainability Our Offering
Our Offering Our History
Our History Our Locations
Our Locations Leadership
Leadership