Over the course of my career, I have had terrific interactions with a multitude of chemists discussing chromatography. When it comes to flash chromatography the approaches from these chemists ranged from running generic 0-100% ethyl acetate in hexane gradients to modeled gradients based on tribal knowledge for a type of synthetic molecule to always using TLC for method development to prep HPLC. Each of these techniques are used because they provide some level of success. To me, though, if I spend time and resources synthesizing a highly valuable and unique molecule, then I want to purify the reaction mixture with the best possible method.
What is best is what provides you the most consistent, reliable results. It has been my experience that taking a few minutes to run TLCs with different solvents and different solvent ratios can really pay off in terms of increased compound purity, yield, and even solvent reduction.
As an example, I synthesized a compound and evaluated its purification comparing a generic 0-100% ethyl acetate gradient against several TLC-based methods, both linear and step gradients. For each purification run, 20 mg was loaded.
The generic 0-100% ethyl acetate gradient provided a decent purification using a 10-gram Biotage® Sfär silica column, Figure 1.
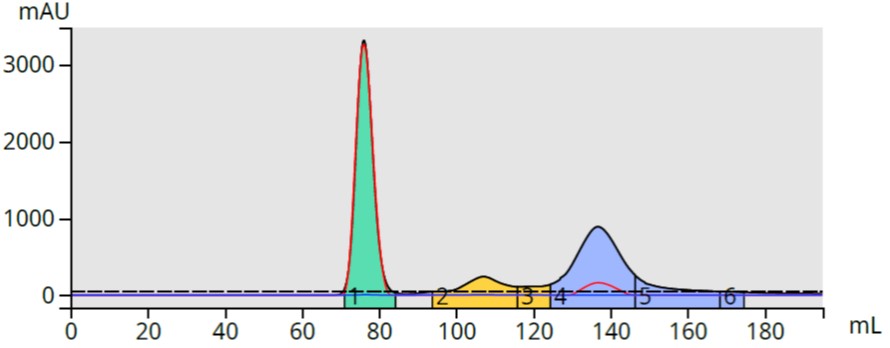
Figure 1. Reaction mixture purification using a generic 0-100% ethyl acetate in hexane gradient provided a good separation of the product (blue) with some by-product contamination in fraction 3 (yellow fraction at ~120 mL).
This method, as with all of the linear gradient methods used for this comparison, was 13 column volumes (CV) or 195-mL long. I mention this because part of method optimization is solvent use minimization.
TLC of the reaction mix was performed in 20%, 30%, and 40% ethyl acetate/hexane yielding many spots. Data from each of the spots was used to create specific linear gradients, Table 1.
Table 1. Reaction mixture Rf values with different ethyl acetate (EA)/hexane ratios.
20% EA 30% EA 40% EA
By-product 1 0.18 0.30 0.46
Product 0.11 0.18 0.29
By-product 2 0.04 0.06 0.08
The 20% ethyl acetate TLC results created a 5-40% ethyl acetate linear gradient (13 CV) and yielded a completely baseline resolved purification, Figure 2. Because of the product's late elution near the end of the programmed 13 CV method, the purification run was automatically extended by the Biotage® Selekt flash system consuming nearly 240 mL.
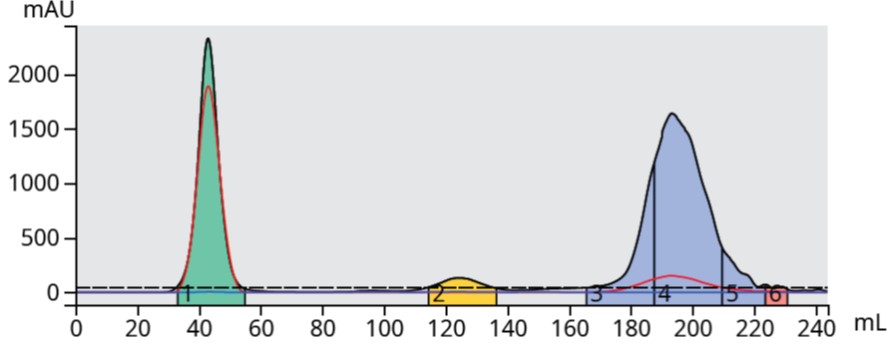
Figure 2. TLC-based 5-40% ethyl acetate in hexane gradient fully separated the product (blue) from its by-products but required 240-mL to complete.
The 30% ethyl acetate TLC results created a 7-60% ethyl acetate gradient forcing the compounds to elute earlier with less separation. But because the TLC to linear gradient software always creates a 13 column volume gradient, the purification volume was the same as the generic gradient (195 mL), Figure 3.
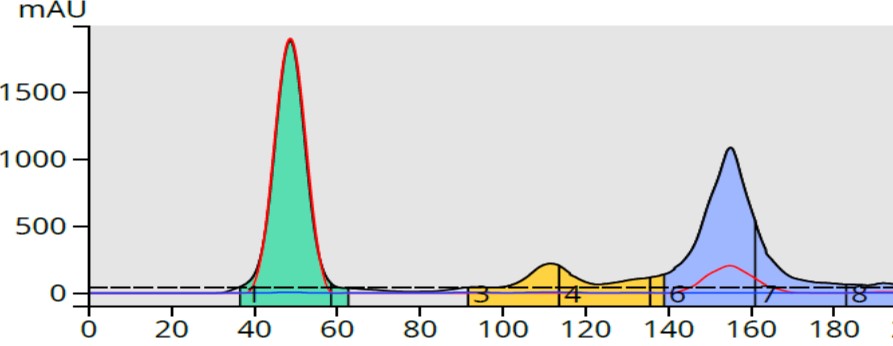
Figure 3. TLC-based 7-60% ethyl acetate in hexane gradient eluted the product (blue) but with some by-product contamination in fractions 4 and 5 (yellow).
The 40% ethyl acetate the TLC results also created a 195-mL long method (10-80% ethyl acetate) with results similar to the 7-60% EA method but with earlier compound elution, Figure 4.
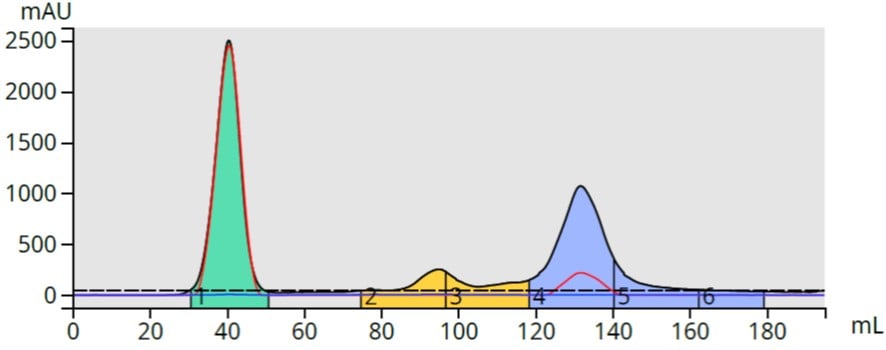
Figure 4. TLC-based 10-80% ethyl acetate gradient provided the same separation as the 7-60% gradient while consuming a similar amount of solvent.
While all these TLC-based linear gradients provided better purifications than the generic 0-100% gradient, especially the 5-40% method, they all consume more solvent than should be necessary. If saving solvent is important to you, a step gradient based on TLC data from two plates can provide as good a separation as a linear gradient, if not better, but using a fraction of the solvent.
Biotage offers two flash systems capable of creating optimized step gradients from two sets of TLC data (two plates) - Biotage® Selekt and Biotage® Isolera. From a user perspective just replicate your TLC results on the touch screen, Figure 5.
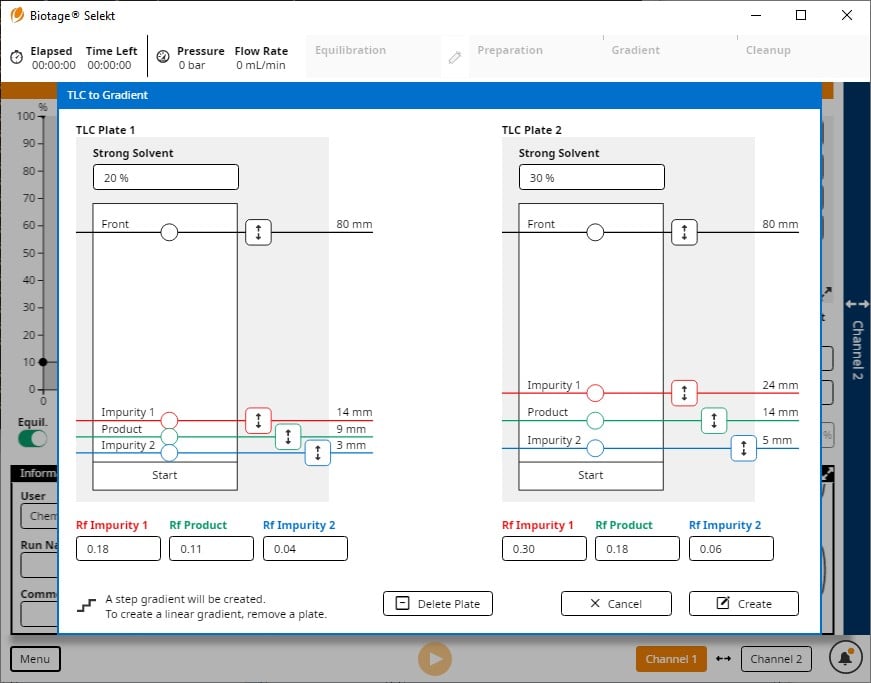
Figure 5. Biotage Selekt TLC to Step gradient interface converts TLC Rf values and solvent % into an optimized purification method.
Using the same TLC data as an example, a step gradient based on the 20% and 30% ethyl acetate TLC runs above, generated an 11-column volume, 3-step gradient, Figure 6.
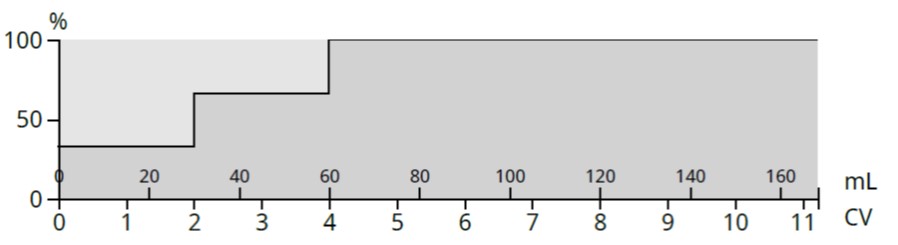
Figure 6. Step-gradient created from Rf data from the 20% and 30% TLC plates.
This step gradient yielded the purification results in Figure 7 where only 90-mL was required to fully separate the product from its by-products.
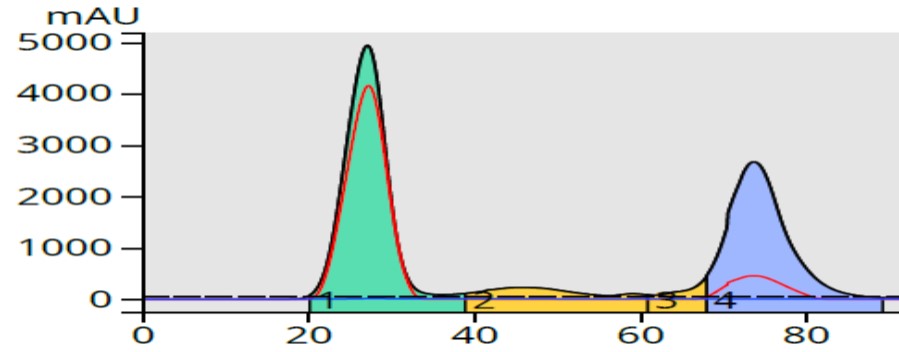
Figure 7. TLC-based step gradient created from the 20% and 30% ethyl acetate/hexane Rf data provided a better separation than all but the 5-40% linear gradient, but consumed about half of the solvent.
So, while a generic, non-optimized purification gradient can provide suitable results, spending a little time scouting TLC solvents and conditions to generate a step gradient will yield better, optimized purification results using less solvent.
Interested in learning more about optimization? Download our white paper – Inspiring Productivity with Modern Flash Chromatography.

 Organic Workflow
Organic Workflow Peptide Workflow
Peptide Workflow Scale-Up Flash Purification
Scale-Up Flash Purification  Sample Preparation
Sample Preparation Biomolecule Purification
Biomolecule Purification Oligo synthesis
Oligo synthesis Scavengers and Reagents
Scavengers and Reagents Service & Support
Service & Support Accessories & Spare parts
Accessories & Spare parts Investors
Investors Reports & News
Reports & News The Share
The Share Corporate Governance
Corporate Governance Calendar
Calendar Sustainability
Sustainability Our Offering
Our Offering Our History
Our History Our Locations
Our Locations Leadership
Leadership
